Investors & News
Our News
- NOTITIA BIOTECHNOLOGIES UNVEILS GROUNDBREAKING MICROBIOME DISCOVERY: A CORE MICROBIOME SIGNATURE AS A NEW HEALTH INDICATOR
- NOTITIA BIOTECHNOLOGIES ANNOUNCES EARLY COMPLETION OF PHASE 2 STUDY COVGUT20 FOR NBT- NM108
- NOTITIA BIOTECHNOLOGIES COMPANY ANNOUNCES EXPANSION OF STUDY POPULATION FOR COVID-19 PHASE 2 STUDY (COVGUT20) FOR NBT-NM108
- RUTGERS RESEARCHER INVENTS MICROBIOTA FORMULA TO HELP HIGH-RISK PATIENTS FIGHT COVID-1
- NOTITIA BIOTECHNOLOGIES RECEIVES APPROVAL FROM FDA FOR PHASE 2A CLINICAL TRIAL IN DIABETIC & PREDIABETIC PATIENTS
- NOTITIA BIOTECHNOLOGIES DEVELOPS REVOLUTIONARY FOUNDATION GUILD LBP FOR PATIENTS WITH DIABETIC KIDNEY DISEASE (DKD)
Notitia Biotechnologies Unveils Groundbreaking Microbiome Discovery: A Core Microbiome Signature as a New Health Indicator
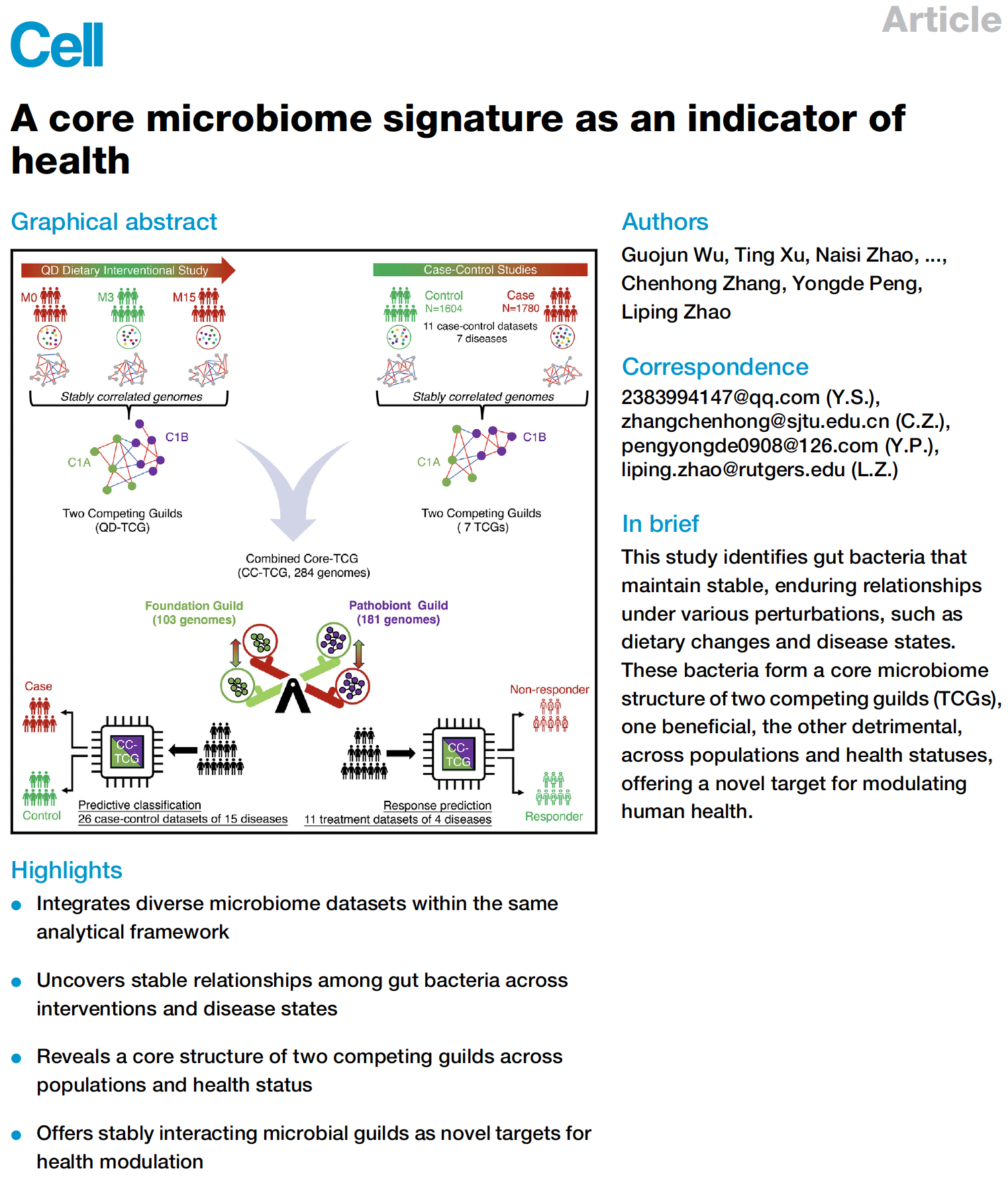
Monmouth Junction, NJ, October 7th , 2024 – Notitia Biotechnologies, a leader in microbiome-based therapies, announces the publication of a pivotal study in Cell (2024) by its co-founder, Dr. Liping Zhao, and an international team of collaborators. This landmark research identifies a "Core Microbiome Signature" composed of two key groups of bacteria—the Foundation Guild™ and Pathobiont Guild—which serve as crucial indicators of gut health.
Utilizing the proprietary Two Competing Guilds (TCG) model, the study reveals a dynamic "seesaw" relationship between these bacterial groups, enabling accurate prediction of individual health status across various demographics. This discovery opens new avenues for personalized medicine, microbiome restoration therapies, and next-generation health diagnostics.
Empowering Health Through Gut Microbiome Analysis
At the heart of this breakthrough discovery is the Foundation Guild, a group of beneficial bacteria responsible for fiber fermentation and producing short-chain fatty acids (SCFAs), essential for metabolic and immune health. The Pathobiont Guild, in contrast, includes bacteria associated with inflammation and disease.
“This study provides scientific validation for our novel microbiome therapy, which aims to restore the balance between these two bacterial guilds to enhance health and prevent disease,” says Jeffrey Zhao, CEO of Notitia Biotechnologies. “Our innovative approach is setting new standards for gut microbiome therapies and disease prevention.”
Targeted Nutrition and Dietary Products: Enhancing the Foundation Guild
Building on this groundbreaking discovery, Notitia is expanding its research into targeted nutrition to promote the growth of the Foundation Guild bacteria. The company’s existing microbiome nutrition product, Guild:Plus, plays a key role in this effort. Guild:Plus is specifically designed to nurture Foundation Guild bacteria through a blend of prebiotics and fibers known to enhance their function, helping to restore gut health in individuals experiencing dysbiosis or imbalance in their microbiota.
Dr. Liping Zhao adds, “Our product development is focused on creating precision nutrition solutions that target the Foundation Guild. Through targeted dietary interventions, such as Guild:Plus, we aim to enrich the beneficial bacteria essential for gut health. These products will give consumers a powerful tool for promoting a healthy microbiome.”
Redefining Health with the Two Competing Guilds Model
Dr. Zhao’s research directly applies to Notitia’s therapeutic pipeline, including the company’s NBT-NM108 and pipelines of other novel microbiome therapies aiming to treat conditions associated with gut dysbiosis. These products are undergoing clinical trials, with plans to demonstrate their potential to address chronic diseases such as type 2 diabetes, inflammatory bowel disease, and more.
“We are advancing the frontier of microbiome science,” Dr. Zhao explains. “By understanding the core microbiome signature, we hope to develop more effective treatments to alleviate a wide range of diseases.”
Nutrition and Health: A Vision for the Future
With the addition of targeted nutrition products like Guild:Plus, Notitia is transforming how the microbiome can be supported through everyday dietary choices. By leveraging insights from the TCG model, Notitia’s products promote the specific growth of health- promoting bacterial guilds while suppressing pathogenic microbes. The ongoing development of these dietary products reflects Notitia’s commitment to improving health outcomes through accessible, nutrition-based solutions.
This breakthrough will be the focus of several upcoming research initiatives and collaborations, with detailed findings and product innovations available on the company’s website.
Notitia Biotechnologies Announces Early Completion of Phase 2 Study COVGUT20 for NBT- NM108
Trial Hits Primary Endpoint Ahead of Schedule: NBT-NM108 Demonstrates Potent Promotion of Foundation Guild™ Bacteria
Monmouth Junction, NJ – July 3rd , 2024 – Notitia Biotechnologies is proud to announce the early completion of its Phase 2 clinical trial COVGUT20 for the investigational drug NBT-NM108. The trial achieved its primary endpoint ahead of schedule, demonstrating that NBT-NM108 effectively promotes the gut bacteria Amplicon Sequence Variants (ASVs) that are members of the Foundation Guild, a core component of the human microbiome essential for gut health.
The COVGUT20 study was designed to assess whether NBT-NM108 could support the growth of these beneficial bacteria, critical for gut microbiota balance and the alleviation of gut dysbiosis, a condition commonly linked to severe gastrointestinal and metabolic disorders. The study randomized participants into treatment and control groups, and those receiving NBT-NM108 displayed a significant increase in the targeted SCFA-producing bacteria, which are part of the Foundation Guild.
“The successful promotion of Foundation Guild bacteria underpins our approach of restoring gut health through ecological balance,” said Dr. Liping Zhao, Co-Founder and Chief Scientific Officer of Notitia Biotechnologies. “The fact that we completed the study ahead of schedule highlights the strong efficacy of NBT-NM108, bringing us closer to offering a groundbreaking treatment for microbiome-related diseases.”
Primary Endpoint: Restoration of Foundation Guild Bacteria
NBT-NM108 is a fiber-based investigational new drug designed to modulate the gut microbiome, specifically targeting and enriching Foundation Guild bacteria. These bacteria play a key role in promoting the production of short-chain fatty acids (SCFAs), crucial for maintaining gut health. The study showed that patients in the treatment arm had a statistically significant increase in these SCFA-producing bacteria, which are known to suppress harmful microbes and support overall gut stability.
Positive Implications for COVID-19 and Other Conditions
The COVGUT20 study was initially launched to explore whether NBT-NM108 could alleviate gut- related complications in patients with COVID-19, but the results suggest broader applications. “By promoting the growth of Foundation Guild bacteria, we are advancing a universal solution for treating gut dysbiosis, which can manifest in multiple diseases including metabolic and inflammatory conditions,” added CEO Gongze "Jeffrey" Zhao.
Looking Forward
With the successful completion of the COVGUT20 study, Notitia is preparing for later-stage trials to further validate NBT-NM108’s efficacy and safety. The company is also exploring additional indications where restoring the Foundation Guild microbiota can improve patient outcomes.
Rutgers Researcher Invents Microbiota Formula to Help High-Risk Patients Fight COVID-19
A Rutgers scientist has invented an early treatment for COVID-19 to prevent severe complications and hospitalizations in patients with prediabetes and diabetes by increasing beneficial bacteria in the gut and reducing organisms that cause coronavirus.
The treatment – created by researcher Liping Zhao – was given the Investigational New Drug status by the Food and Drug Administration and will start Phase 2a trial Feb. 8. While the treatment, NBT-NM108, does not target the coronavirus directly, it does help to create stable foundation for a healthy digestive tract.
“COVID-19 patients with pre-existing conditions such as obesity and type 2 diabetes suffer three to four times higher mortality rate than the average patient population,” said Zhao, professor and Eveleigh-Fenton Chair of Applied Microbiology of the Department of Biochemistry and Microbiology at the School of Environmental and Biological Sciences. “Overgrowth of opportunistic pathogens in the gut of diabetic or prediabetic patients may drive the more severe form of COVID-19. Such pathogens can contribute to the cytokine storm and sepsis, which are the major causes of mortality in these high-risk patients.”
Researchers will begin recruiting patients with diabetes and prediabetes who have tested positive for coronavirus, or are awaiting test results, and are within seven days of onset of COVID-19 symptoms. They hope to have results from the initial study by April.
Zhao has shown in previous studies that proper microbiome nutrition can increase good bacteria in the digestive track of those with type 2 diabetes. When good bacteria were restored, opportunistic pathogens in the gut were reduced to low levels. While the new treatment does not directly target the coronavirus, the scientists believe that a reduction of these pathogens may lead to decreased levels of inflammation and also reduce risk for complications such as bacterial sepsis.
“Controlling the gut microbiota in a way that doesn’t allow the overgrowth of pathogens in the gut could become a very important component of the COVID-19 care,” said Zhao.
The participants for the clinical trial will be recruited by the University of South Florida as part of a collaboration and partnership with Rutgers. The day-to-day measurements and samples will be handled by Liping Zhao’s Lab at Rutgers through an online patient managing platform. The early treatment of the gut microbiota with Zhao’s formula will be a home-based intervention for patients with mild to moderate symptoms.
This clinical trial is sponsored by a Rutgers startup, Notitia Biotechnologies, a next-generation microbiome biotherapeutic company that develops innovative products for patients and the general public.
“We have the vision that everyone can live a long and healthy life by keeping our gut microbiome to the healthiest possible status for the longest possible time. Notitia’s mission is to help everyone achieve a healthy gut microbiota by protecting or restoring the foundation guild of the gut microbial forest,” said Jeffery Zhao, cofounder and CEO of Notitia Biotechnologies.
Notitia Biotechnologies Receives Approval from FDA for Phase 2a Clinical Trial in Diabetic & Prediabetic Patients
Notitia Biotechnologies Company has received the Investigational New Drug (IND) approval from the FDA for NBT-NM108, a proprietary formula designed to support the Foundation Guild bacteria in the patient's gut microbiome. The FDA has allowed Notitia to test the drug as a treatment for COVID-19 in patients with type 2 diabetes and prediabetes. Notitia has secured funding for a Phase 2a randomized, controlled clinical trial, and the study will be performed in the Tampa Florida area. Enrollment is expected to begin by October, and further information regarding the study is available on clinicaltrials.gov.
For more information regarding Notitia's Foundation Guild Microbiome R&D Platform, please click here. For more information about the NBT-NM108's development pipeline, please click here.